Blue Earth Diagnostics Announces Addition of POSLUMA (Flotufolastat F 18) Injection to NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Prostate Cancer
Imaging Technology
JULY 28, 2023
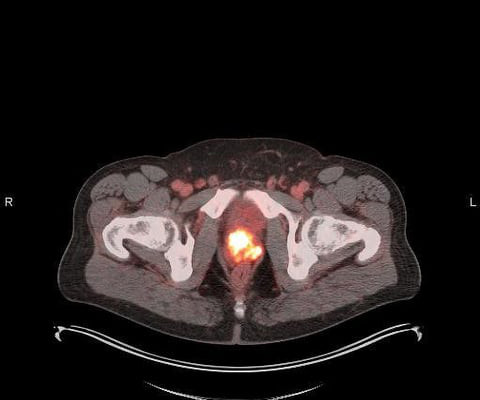
We believe it further validates the clinical utility of POSLUMA in patients with newly diagnosed or recurrent prostate cancer, and can help expand patient access. FDA, as well as on the Phase 3 clinical trial results published recently in the Journal of Urology and European Urology. Chief Executive Officer of the Company. “We











Let's personalize your content