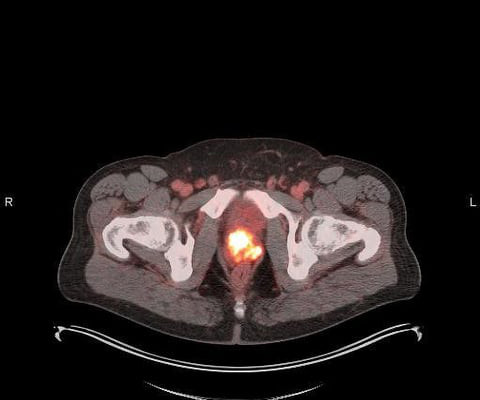
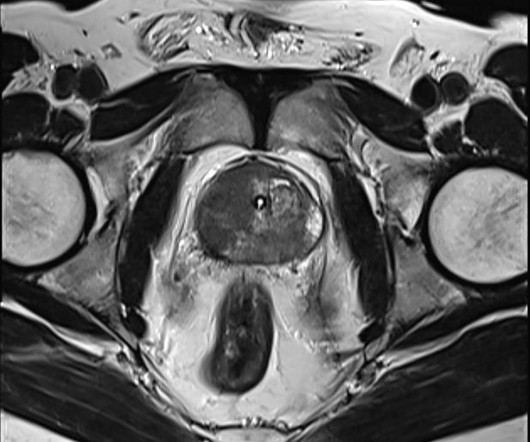
Blue Earth Diagnostics Announces Addition of POSLUMA (Flotufolastat F 18) Injection to NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Prostate Cancer
Imaging Technology
JULY 28, 2023
The addition of POSLUMA to the highly respected NCCN Guidelines is a major milestone for Blue Earth Diagnostics,” said David E. We believe it further validates the clinical utility of POSLUMA in patients with newly diagnosed or recurrent prostate cancer, and can help expand patient access. Chief Executive Officer of the Company. “We












Let's personalize your content