New portable PET scanner tested in humans
AuntMinnie
DECEMBER 19, 2023
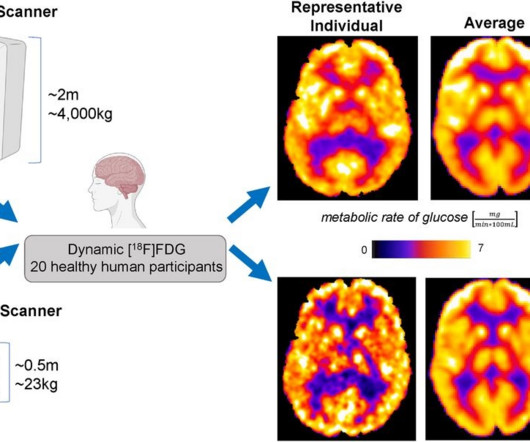
Clinical brain imaging with a newly developed portable PET scanner appears feasible, with a comparison to conventional PET imaging for the first time revealing similar results in human subjects, according to a group in New York City. In 2018, the group received a grant from the U.S.









Let's personalize your content