Blue Earth Therapeutics Announces Promising Results from Preclinical Evaluation of Synergistic Drug Combinations with Radiopharmaceutical 177Lu-rhPSMA-10.1 for Treatment of Prostate Cancer
Imaging Technology
APRIL 10, 2024
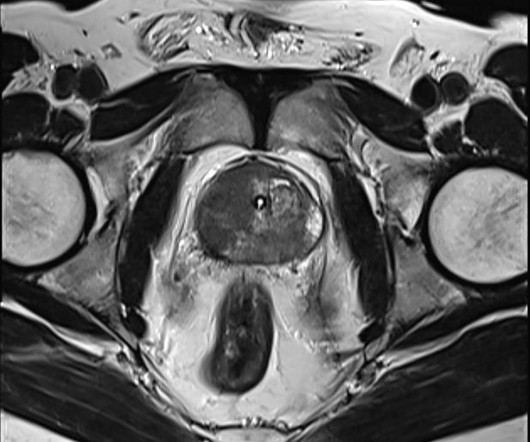
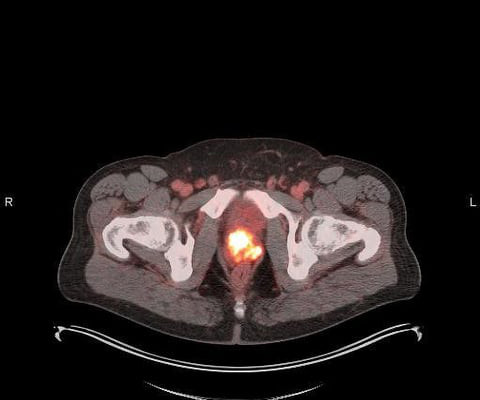
an investigational radiohybrid (rh) Prostate-Specific Membrane Antigen-targeted therapeutic radiopharmaceutical, is the lead candidate in Blue Earth Therapeutics’ oncology development program of next generation therapeutic radiopharmaceuticals. Our ongoing Phase 1/2 clinical trial ( NCT05413850 ) is evaluating 177Lu-rhPSMA-10.1















Let's personalize your content