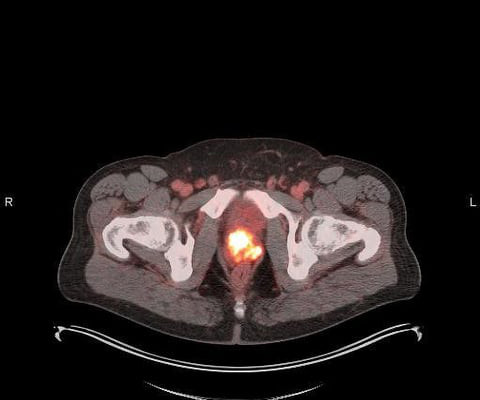
Additional Results of POSLUMA (Flotufolastat F 18) Performance in Newly Diagnosed, High-risk Prostate Cancer Patients Presented at ASTRO
Imaging Technology
OCTOBER 5, 2023
Specifically, this sub-group examined the performance of flotufolastat F 18 PET in newly diagnosed, high-risk prostate cancer patients who had negative results with conventional imaging. Kuo, MD, Ph.D. , Departments of Medical Imaging, Medicine, and Biomedical Engineering. Recently approved by the U.S. on behalf of Gary A.











Let's personalize your content