Pharmacist sentenced in radiopharmaceutical criminal case
AuntMinnie
JULY 26, 2024
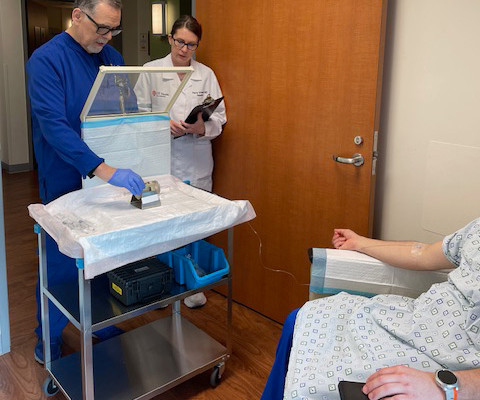
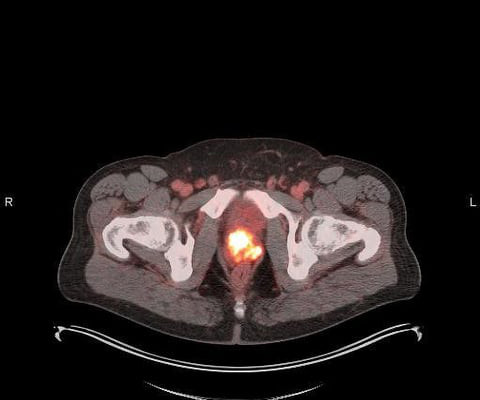
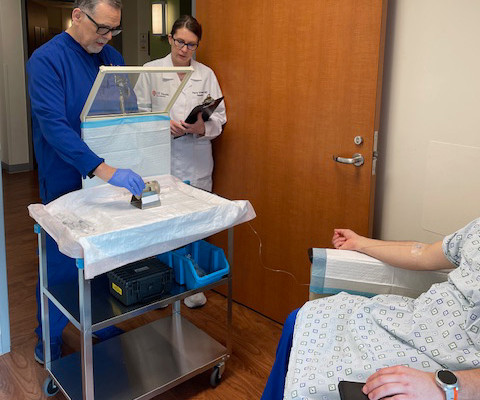
Evidence showed that Sheriff owned and was pharmacist in charge at Shertech, a pharmacy that provided nuclear and radiopharmaceutical drugs to medical facilities. The resulting diluted product was used in procedures such as renal scans to diagnose various illnesses, such as kidney disease.












Let's personalize your content