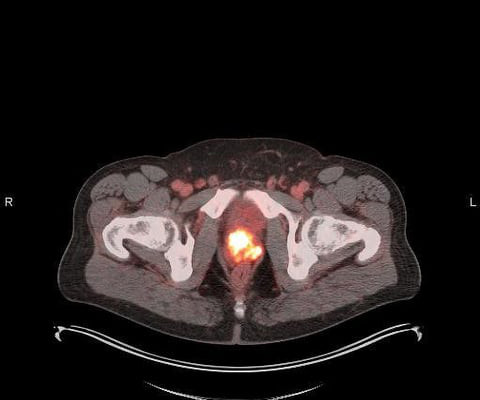
U.S. FDA Approves Blue Earth Diagnostics’ POSLUMA (Flotufolastat F 18) Injection, First Radiohybrid PSMA-targeted PET Imaging Agent for Prostate Cancer
Imaging Technology
MAY 30, 2023
milla1cf Tue, 05/30/2023 - 19:49 May 30, 2023 — Blue Earth Diagnostics , a Bracco company and recognized leader in the development and commercialization of innovative PET radiopharmaceuticals , today announced U.S. Chief Executive Officer of Blue Earth Diagnostics. Gauden , D.Phil.,








Let's personalize your content