Targeted radiopharmaceuticals offer new hope for longer 'healthspans'
AuntMinnie
MARCH 15, 2024
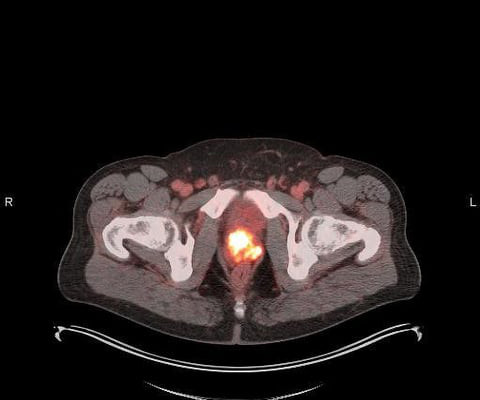
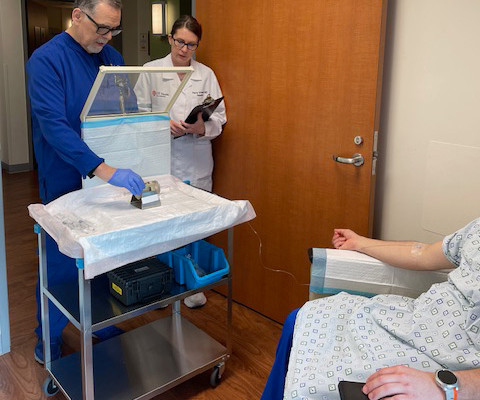
A 2018 study from the U.S. An emerging field of precision radiopharmaceuticals brings new optimism to this mission, revolutionizing cancer care. Precision radiopharmaceuticals accurately target tumors while minimizing damage to healthy tissue. Americans today can expect to live long lives.












Let's personalize your content