The rise of theranostics: Part 1 -- Gaining momentum
AuntMinnie
MARCH 26, 2024
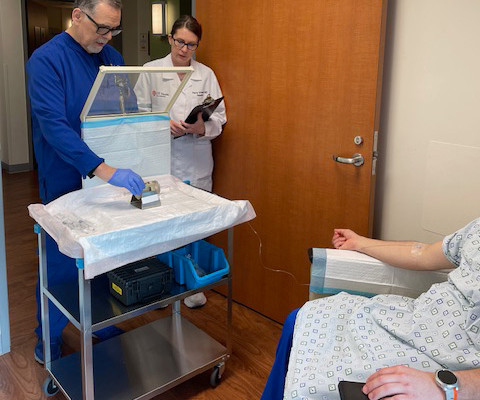
Theranostics pairs diagnostic biomarkers that can be visualized on nuclear medicine imaging with therapeutic agents that share a specific target in diseased cells or tissues. Lutathera was approved for adults by the FDA in 2018 after the therapy resulted in progression-free survival (median overall survival of 11.7








Let's personalize your content