New portable PET scanner tested in humans
AuntMinnie
DECEMBER 19, 2023
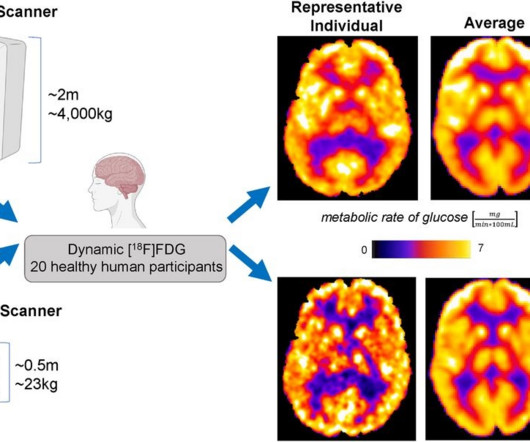
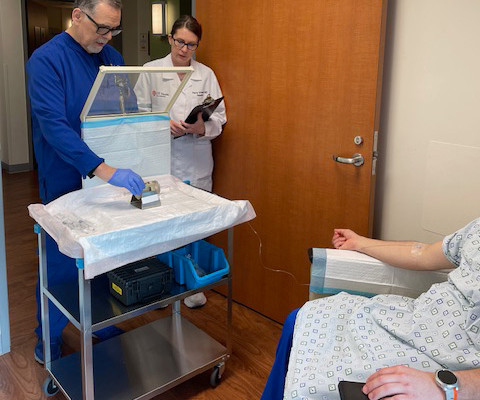
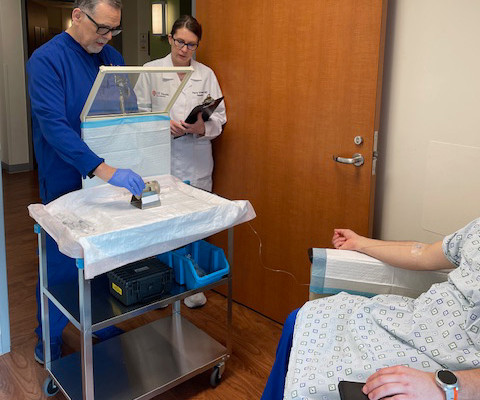
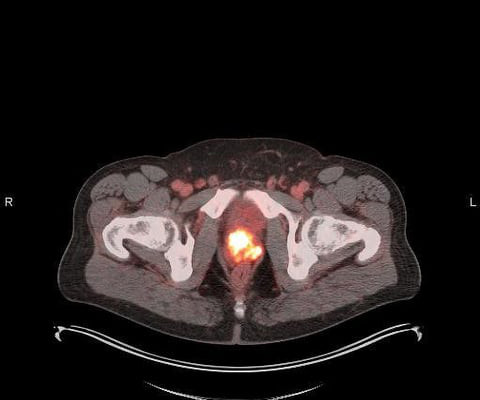
Conversely, recently developed portable PET scanners include seated or standing configurations and have been proposed for use in combat zones, sports fields, and intensive care units, the researchers added. In 2018, the group received a grant from the U.S. A link to the full study can be found here.









Let's personalize your content