The rise of theranostics: Part 1 -- Gaining momentum
AuntMinnie
MARCH 26, 2024
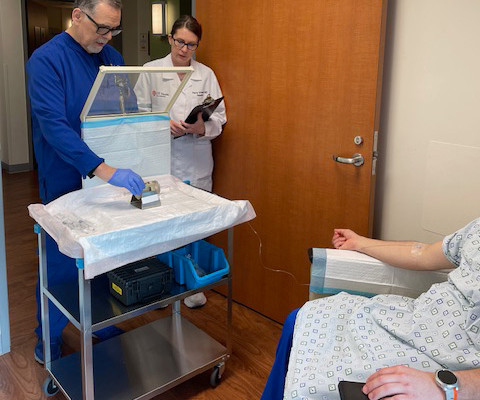
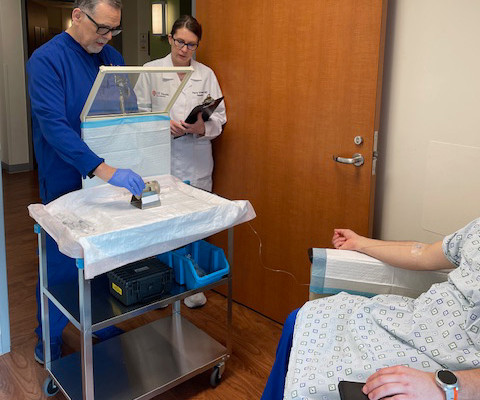
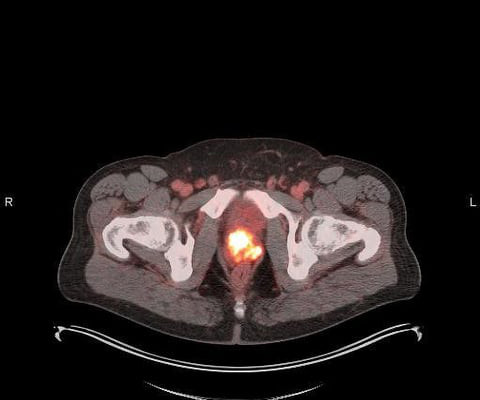
The L block lead shield over the syringe helps protect certified nuclear medicine technologist James Johnson (left), and nuclear radiologist Penny Vroman, MD (right), from radiation emitted by the drug. After binding to the receptor, the drug works by entering the cell allowing radiation to cause damage to the tumor cells.









Let's personalize your content