First Ever Investigational 18F-CD8 PET Radiopharmaceutical Aims to Predict and Monitor Early Response to Cancer Immunotherapies
Imaging Technology
JUNE 15, 2023
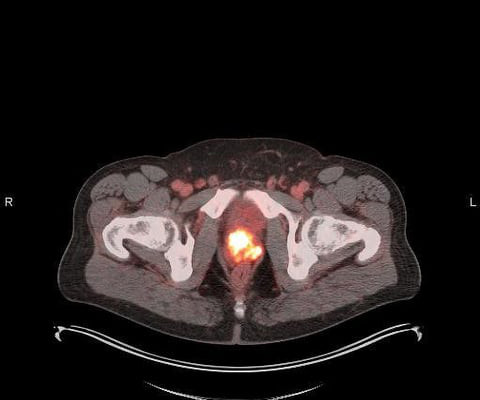
The clinical trial will use this investigational radiopharmaceutical to help understand if patients have CD8+ T cells in their tumors and will, therefore, be more likely to respond to immune checkpoint inhibitors, the main class of immunotherapies currently approved for use. Fruhwirth, G. Kneilling, M., De Vries, I. Weigelin, B.,








Let's personalize your content