Clarity touts FDA fast track designation
AuntMinnie
AUGUST 22, 2024
Food and Drug Administration (FDA) has granted Clarity Pharmaceuticals fast-track designation for its copper-64 (Cu-64) sarcophagine (SAR) bisPSMA radiopharmaceutical.
AuntMinnie
AUGUST 22, 2024
Food and Drug Administration (FDA) has granted Clarity Pharmaceuticals fast-track designation for its copper-64 (Cu-64) sarcophagine (SAR) bisPSMA radiopharmaceutical.

Imaging Technology
JULY 28, 2023
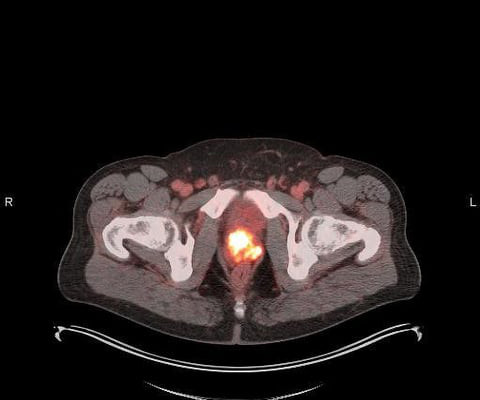
POSLUMA is indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer with suspected metastasis who are candidates for initial definitive therapy or with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Imaging Technology
APRIL 2, 2024
The availability of these offerings – including the MIM SurePlan and MIM Symphony families, MIM Maestro, MIM Encore, and more – is in alignment with GE HealthCare’s precision care strategy, which aims to deliver innovative digital solutions across care pathways for more precise, connected, and efficient care across disease states. "We
Imaging Technology
MAY 30, 2023
milla1cf Tue, 05/30/2023 - 19:49 May 30, 2023 — Blue Earth Diagnostics , a Bracco company and recognized leader in the development and commercialization of innovative PET radiopharmaceuticals , today announced U.S. In clinical trials , the safety of POSLUMA was evaluated in 747 patients with initial or recurrent prostate cancer.

Imaging Technology
SEPTEMBER 27, 2023
POSLUMA is indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer with suspected metastasis who are candidates for initial definitive therapy or with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level. “PET Gauden , D.Phil.,

Imaging Technology
OCTOBER 5, 2023
FDA, POSLUMA is indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer with suspected metastasis who are candidates for initial definitive therapy or with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level.

AuntMinnie
JUNE 3, 2024
With therapeutic radiopharmaceuticals coming to market quickly and available to smaller institutions around the U.S., My vision of a theranostic center includes a flagship facility that has a PET/CT scanner, a SPECT/CT scanner, and then multiple rooms for infusions of radiopharmaceuticals," Siegel explained.
Let's personalize your content