Perspective Therapeutics Announces Exclusive License Agreement with Mayo Clinic for New Radiopharmaceutical Platform for Prostate Cancer Treatment
Imaging Technology
JANUARY 5, 2024
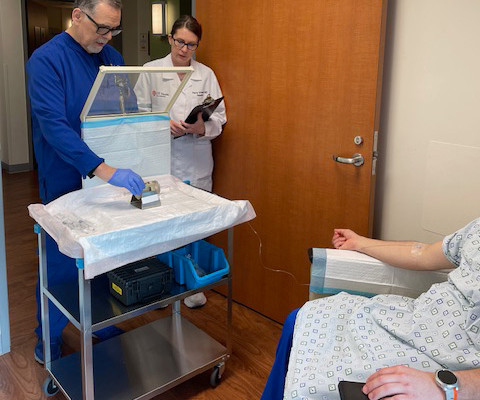
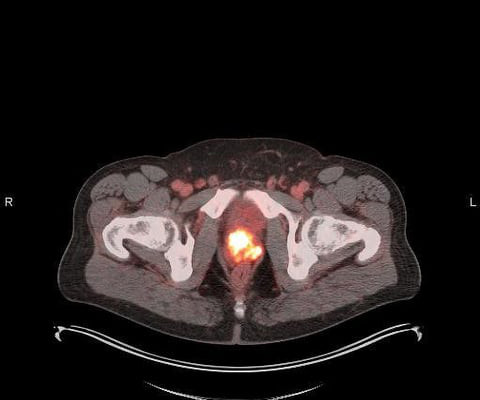
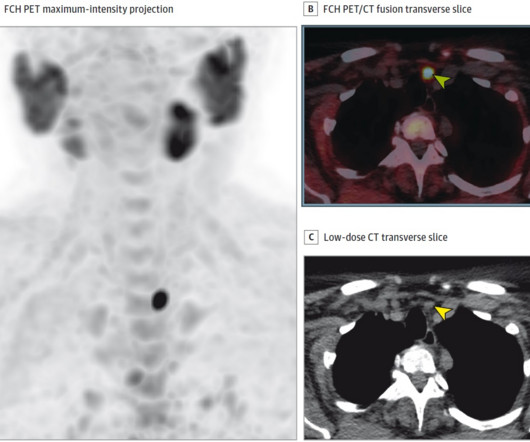
announced that it has entered into a patent license agreement with Mayo Clinic for the rights to the PSMA Alpha-PET DoubLET platform technology for the treatment of PSMA-expressing cancers, with an initial focus on prostate. We focused on diagnostic accuracy, patient tolerance and convenience.






















Let's personalize your content