ASTRO: Lu-177 dotatate shows promise for treating meningioma patients
AuntMinnie
SEPTEMBER 30, 2024
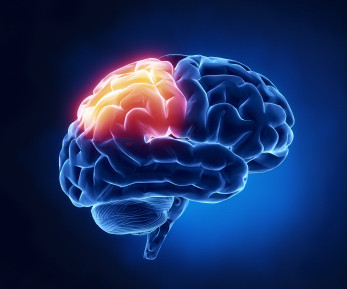
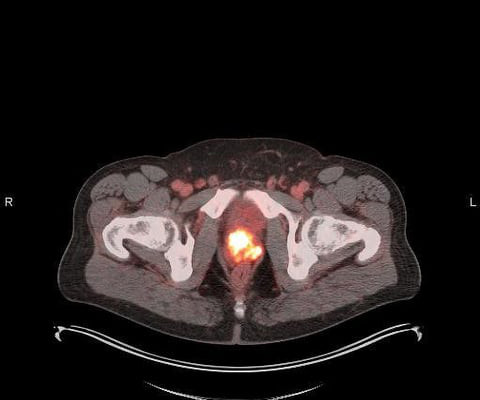
A radiopharmaceutical therapy that lengthens progression-free survival for patients with neuroendocrine cancer could help treat meningioma sufferers, according to research presented at the American Society for Radiation Oncology (ASTRO) meeting. The researchers also tracked overall survival and rates of adverse events.












Let's personalize your content