FDA defines 'transparency' for AI medical devices
AuntMinnie
FEBRUARY 1, 2024
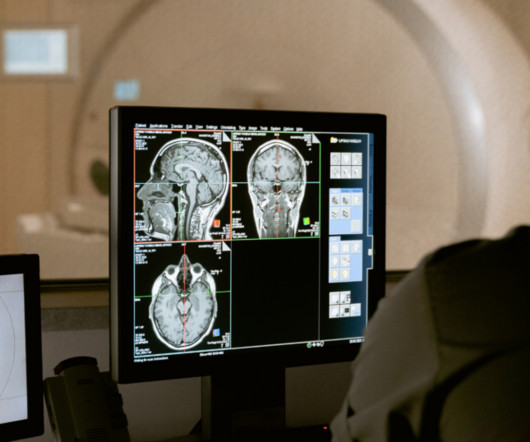
“Transparency is the degree to which appropriate information about a device – including its intended use, development, performance, and, when available, logic – is clearly communicated to stakeholders,” the group wrote. The full article is available here.






















Let's personalize your content