Blue Earth Diagnostics Highlights Presentations on POSLUMA (Flotufolastat F 18) in Prostate Cancer at Upcoming ASTRO Annual Meeting
Imaging Technology
SEPTEMBER 27, 2023
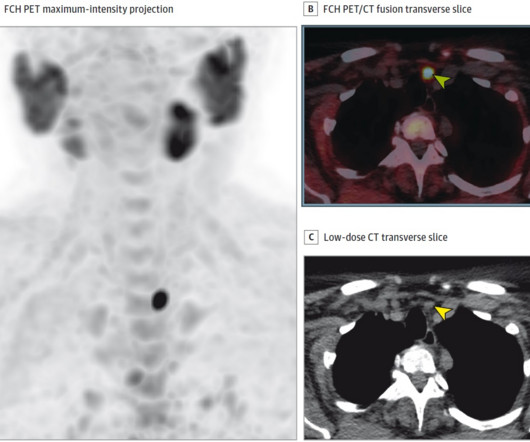
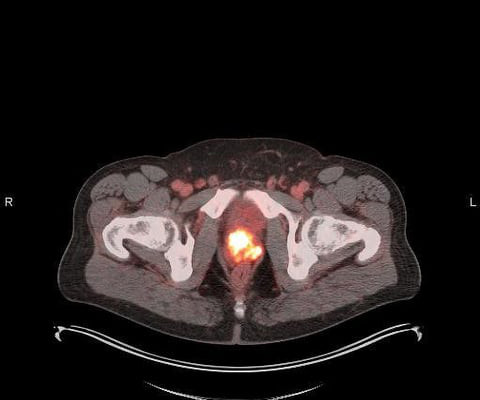
PET imaging with POSLUMA reveals clinical information crucial to decision-making for men with prostate cancer, and we are excited to share further information with the radiation oncology community at ASTRO 2023,” said David E. Chief Executive Officer of Blue Earth Diagnostics. from October 1 to 4, 2023. “PET Gauden , D.Phil.,











Let's personalize your content