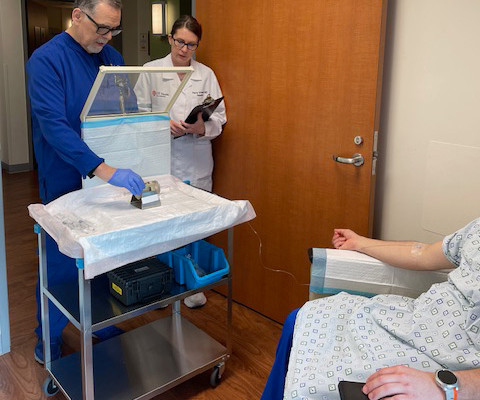
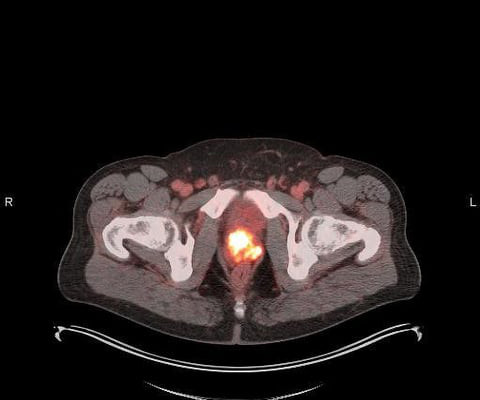
Additional Results of POSLUMA (Flotufolastat F 18) Performance in Newly Diagnosed, High-risk Prostate Cancer Patients Presented at ASTRO
Imaging Technology
OCTOBER 5, 2023
Specifically, this sub-group examined the performance of flotufolastat F 18 PET in newly diagnosed, high-risk prostate cancer patients who had negative results with conventional imaging. We are pleased to present these results from the LIGHTHOUSE study to the radiation oncology community at ASTRO,” said David E. Gauden, D.Phil.












Let's personalize your content