GE HealthCare Announces Agreement to Acquire MIM Software
Imaging Technology
JANUARY 8, 2024
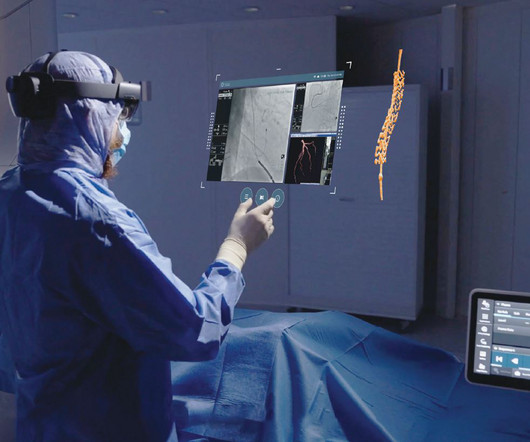
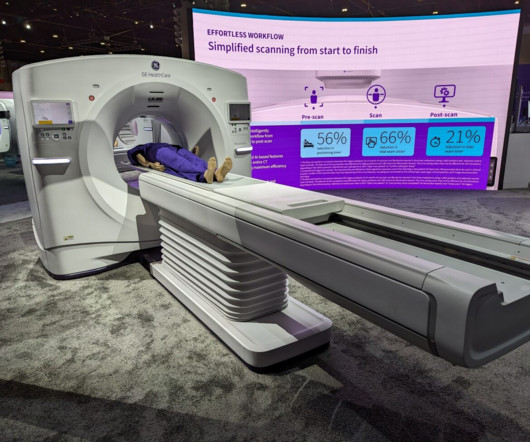
GE HealthCare expects to leverage MIM Software’s imaging analytics and digital workflow capabilities across various care areas to accelerate innovation and differentiate its solutions for the benefit of patients and healthcare systems around the world.
















Let's personalize your content