Intellectt, Inc. Acquires U.S. Electronics, Inc. to Bolster Medical Device Manufacturing and Staffing Expertise
Imaging Technology
NOVEMBER 15, 2023
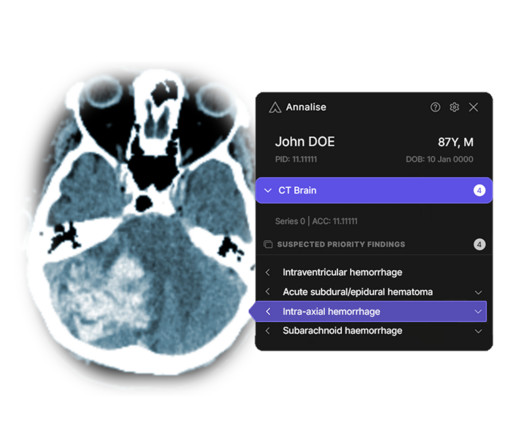
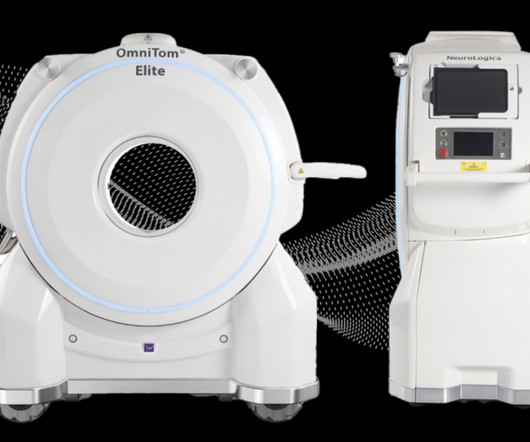
USEI), a reputable medical device manufacturing company with over 25 years of experience announced it has been acquired by Intellectt, Inc., Founded in 1994, USEI is an ISO 13485-certified medical device manufacturing company specializing in medical monitors and accessories. Electronics, Inc. Intellectt, Inc.



















Let's personalize your content