ARRS: How to manage the side effects of radiopharmaceuticals
AuntMinnie
MAY 8, 2024
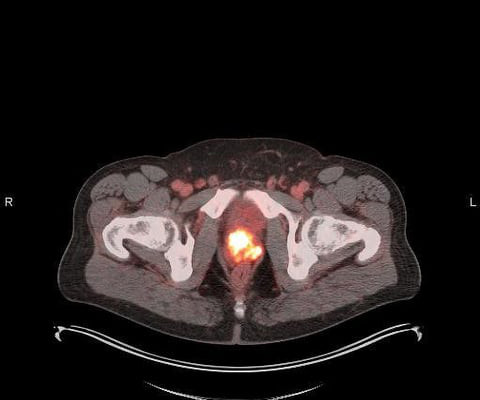
Theranostics treatments are used with increasing frequency now since the approvals and expansions of Lutathera (lutetium-177 [Lu-177]) DOTATATE, a radiopharmaceutical for neuroendrocrine tumors (NETs) and Pluvicto, Lu-177 PSMA-617, for prostate cancer. Extravasations Extravasations, in particular, are of interest to the U.S.












Let's personalize your content