FDA warns against use of Hologic breast care device after nearly 200 adverse events
Radiology Business
OCTOBER 27, 2024
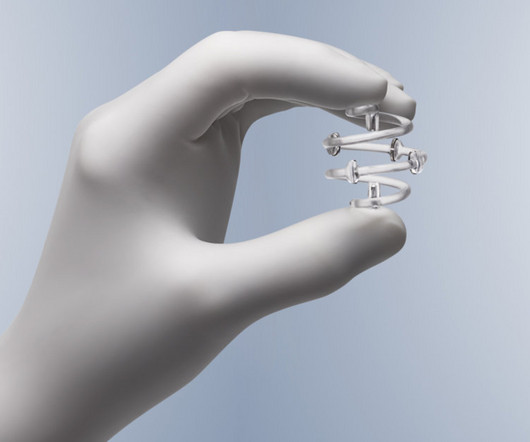
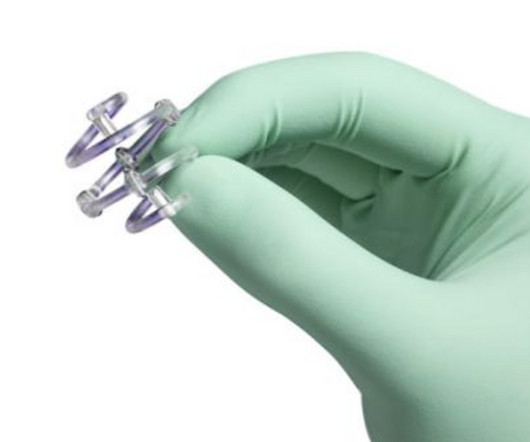
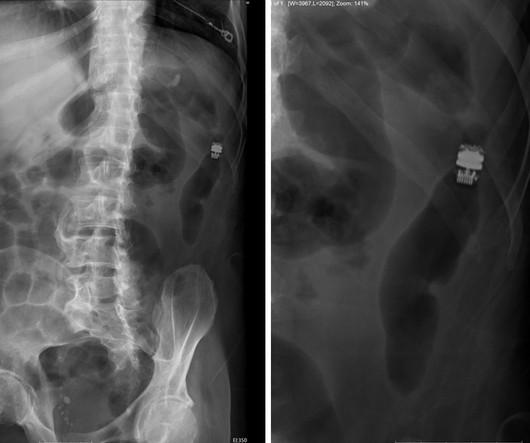
The alert pertains to the BioZorb and BioZorb LP markers, implanted in soft tissue to indicate the site for radiographic procedures.






























Let's personalize your content