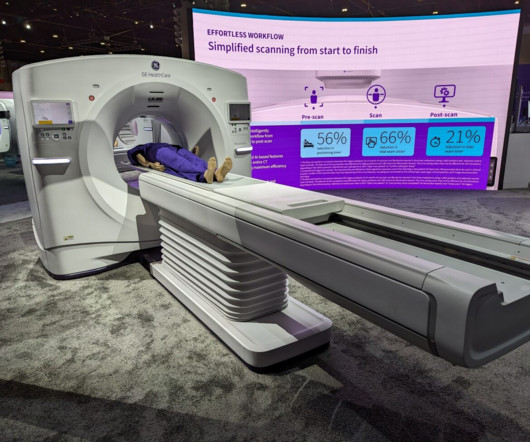
New CT, MR scanners and AI top GE HealthCare highlights at RSNA 2023
AuntMinnie
DECEMBER 1, 2023
Food and Drug Administration (FDA) 510(k) clearance, is designed to support vendor-agnostic remote scanning capabilities As a result of the new technology, remote clinical users can currently initiate a scan on GEHC’s MR devices from inside or outside the radiology suite or at any clinical facility, according to the firm.







Let's personalize your content