Calif. exemption changes nuclear medicine tech scope of practice
AuntMinnie
JANUARY 10, 2025
The state of California now allows certified nuclear medicine technologists to administer any radiopharmaceuticals for therapeutic purposes.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

AuntMinnie
JANUARY 10, 2025
The state of California now allows certified nuclear medicine technologists to administer any radiopharmaceuticals for therapeutic purposes.

Radiology Business
OCTOBER 31, 2024
Leaders said the funding will help Blue Earth to further advance its development of PSMA-targeted therapies for prostate cancer.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Radiology Business
SEPTEMBER 10, 2024
NRC estimates that 29 of these “medical events” occurred between 2021 and 2023, with “many” involving new therapeutic radiopharma procedures.
Radiology Business
MAY 3, 2024
The Swiss drugmaker said the deal will bolster its research infrastructure and clinical supply capabilities, with an eye toward improving oncologic care.

AuntMinnie
MAY 8, 2024
Theranostics treatments are used with increasing frequency now since the approvals and expansions of Lutathera (lutetium-177 [Lu-177]) DOTATATE, a radiopharmaceutical for neuroendrocrine tumors (NETs) and Pluvicto, Lu-177 PSMA-617, for prostate cancer.

Radiology Business
MAY 21, 2024
The Indianapolis-based drugmaker can hit the hefty total by reaching certain clinical, regulatory and commercial milestones.

Radiology Business
OCTOBER 7, 2024
Founded in 2019, the firm is focused on developing products for the diagnosis and therapy of blood cancers and other indications not addressed by nuclear medicine.

Radiology Business
OCTOBER 1, 2024
The Boston clinical stage biotechnology company is working to develop new products to treat a range of solid tumors.

Radiology Business
JULY 12, 2024
For years, the agency has packaged payment for nuclear imaging agents with the procedure, but this has created a barrier for those who require pricier new drugs.

AuntMinnie
NOVEMBER 12, 2024
With 100% of precincts now reporting, we’re finally ready to declare this year’s winners in our annual awards program recognizing excellence in radiology. The voters also zeroed in on the ongoing shortage of radiologists as the Biggest Threat to Radiology. Most Influential Radiology Researcher Minnies 2024 Winner: Erik H.

Radiology Business
JUNE 28, 2024
One of the largest radiopharma companies in the world is acquiring global rights to a pair of novel therapeutic and diagnostic drugs used to target a peptide receptor overexpressed in prostate and breast cancers.

Radiology Business
NOVEMBER 20, 2024
The Australian company will secure exclusive worldwide rights to a suite of diagnostic and therapeutic candidates geared toward urological cancers.

Radiology Business
SEPTEMBER 23, 2024
RLS operates America’s only Joint Commission-accredited radiopharmacy network, with 31 locations covering over 85% of the U.S. population.

Radiology Business
SEPTEMBER 11, 2023
The company has developed a portfolio of nuclear medicine solutions including "lead candidate" MC-339, used for treating small cell lung cancer.

AuntMinnie
FEBRUARY 26, 2025
Additionally, modalities like MRI, CT, bone scans, and PET-CT are significant contributors to greenhouse gas emissions, with radiology accounting for approximately 1% of global healthcare-related emissions. The analysis might help to establish a model for balancing clinical and environmental priorities in radiology departments globally.

Radiology Business
OCTOBER 18, 2023
Nucleus RadioPharma will use the money to establish manufacturing facilities—with one located in Rochester, Minnesota, near Mayo—and build new technology for distribution.

Radiology Business
JULY 14, 2023
The agency issued its solicitation as part of the 2024 Hospital Outpatient Prospective Payment System proposed rule, released late Thursday.
Radiology Business
JULY 23, 2024
The Melbourne, Australia-based radiopharmaceutical firm plans to use the proceeds to accelerate development of products in its theranostics portfolio

Radiology Business
NOVEMBER 5, 2024
CMS is moving forward with a proposal to issue separate reimbursement for radiopharmaceuticals—a change long lobbied for by the imaging industry.

AuntMinnie
JULY 12, 2024
Reshaping the hospital's nuclear medicine and radiopharmaceutical strategy toward theranostics involves intensive and inclusive planning, Beyder explained at SNMMI. Theranostics offers hospital radiology departments the opportunity to keep more reimbursement dollars in the department, Beyder explained.

AuntMinnie
MARCH 7, 2024
In terms of 2023 spending, the average radiopharmaceutical expenditure per fixed PET site is estimated to be $517,000. In 2023, 38% of PET systems were operated by the PET department, 28% by nuclear medicine, 24% by radiology/imaging, 5% by radiation oncology, and 2% by molecular imaging.

AuntMinnie
JUNE 3, 2024
With therapeutic radiopharmaceuticals coming to market quickly and available to smaller institutions around the U.S., My vision of a theranostic center includes a flagship facility that has a PET/CT scanner, a SPECT/CT scanner, and then multiple rooms for infusions of radiopharmaceuticals," Siegel explained.

AuntMinnie
MAY 1, 2024
Food and Drug Administration (FDA) has approved Novartis' Lutathera (lutetium-177 [Lu-177]) DOTATATE radiopharmaceutical for treatment of pediatric patients who are 12 years old and older with somatostatin receptor (SSTR)-positive gastroentropancreatic neuroendocrine tumors (GEP-NETs).

AuntMinnie
NOVEMBER 1, 2024
After many years of advocacy by the American College of Radiology, the Centers for Medicare and Medicaid Services (CMS) will cover CT colonography (CTC) for colorectal cancer (CRC) screening beginning January 1," the college wrote. The ACR lauded the CMS for the colorectal cancer screening coverage.

AuntMinnie
OCTOBER 25, 2024
Who made it to the final round in the 2024 edition of the Minnies, AuntMinnie.com 's event recognizing excellence in radiology? Most Influential Radiology Researcher Erik Middlebrooks, MD, Mayo Clinic, Jacksonville, FL Erik Middlebrooks, MD. Most Effective Radiology Educator Francis Deng, MD, Johns Hopkins Medicine Francis Deng, MD.

Imaging Technology
JANUARY 5, 2024
This leading radiopharmaceutical platform provides detailed PET imaging-based diagnosis and dosimetry using long-lived copper-64 ( 64 Cu) for imaging and alpha-particle targeted RPT using lead-212 ( 212 Pb). milla1cf Fri, 01/05/2024 - 11:21 January 5, 2024 — Perspective Therapeutics, Inc.

AuntMinnie
JUNE 9, 2024
Welch Award in 2012; the Distinguished Investigator Award from the Academy of Radiology Research in 2014; SNMMI's Paul C. She has co-authored more than 200 articles, focusing on developing radiopharmaceuticals for oncological imaging and therapy, the society said.
AuntMinnie
DECEMBER 8, 2023
Suspicion of malignancy may be prompted by avid uptake of the radiopharmaceutical FDG on PET/CT imaging, according to study results delivered on November 27 at the RSNA meeting. Accurate diagnosis of organizing pneumonia is confounded by the co-existence of [the condition] with lung malignancies," Kim said.

AuntMinnie
APRIL 4, 2024
Life Molecular Imaging (LMI) and Sofie Biosciences have begun offering the Neuraceq ( florbetaben F-18) PET radiotracer produced at Sofie's radiopharmaceutical manufacturing site in Cleveland, Ohio. With this expansion, LMI continues driving commercial sales of Neuraceq around the U.S.
AuntMinnie
MARCH 26, 2024
Food and Drug Administration's (FDA) approvals of radiopharmaceuticals for neuroendocrine tumors and then for prostate cancer, theranostics has picked up momentum in clinical practice, propelled by encouraging research. Since 2013 we've had several more radiopharmaceutical therapies get FDA approval. Since the U.S.
AuntMinnie
FEBRUARY 24, 2025
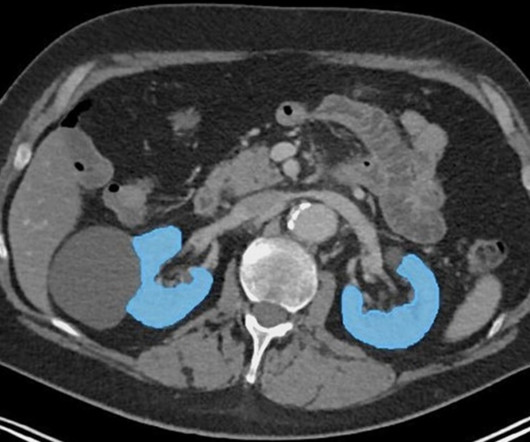
An open-source AI tool can help predict renal function decline in prostate cancer patients undergoing lutetium-177 (Lu-177) radiopharmaceutical therapy, according to a study published February 25 in Radiology.

Open Medscience
MARCH 22, 2024
The post Nuclear Medicine at the Centre of Radiology and Radiopharmaceuticals appeared first on Open Medscience. Nuclear medicine and the future of precise, personalised care in diagnosing and treating diseases.

AuntMinnie
APRIL 15, 2024
According to the report, this year 14% of nuclear medicine sites are currently performing theranostics procedures, with the top two radiopharmaceuticals used being Y-90 microspheres and iodine-131. Changes in nuclear medicine procedure volumes between 2021 and 2023 Facility Type Volume change since 2021 2-year CAGR Hospitals -10.7% -5.5%
AuntMinnie
JANUARY 3, 2024
NorthStar Medical Radioisotopes and Chester Springs, PA-based Curadh MTR have entered a strategic collaboration agreement to develop radiopharmaceuticals for the treatment of primary and metastatic solid tumors.
AuntMinnie
JUNE 20, 2024
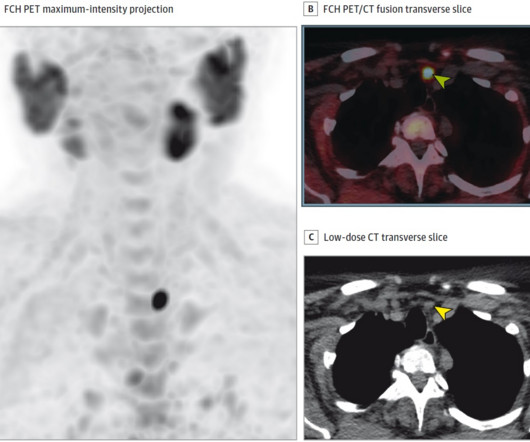
We expect these findings to contribute to the marketing authorization of this existing radiopharmaceutical for a new clinical indication,” it concluded. Additionally, the tracer was found useful in 2014 in patients with PHPT. This study further supports its use in these patients, the team wrote. “We The full study is available here.

AuntMinnie
JUNE 10, 2024
“The approach may also assist in the early management of patients with advanced, end-stage disease by identifying appropriate treatment regimens and predicting response to therapies, such as radiopharmaceutical therapy.” Automatic cancer detection and characterization can make way for early treatment and better patient outcomes.
AuntMinnie
JANUARY 8, 2024
NorthStar will be one of the first commercial-scale producers of actinium-225 for clinical research and commercial radiopharmaceutical therapy, Alpha-9 said. Alpha-9 Oncology and NorthStar Medical Radioisotopes have entered into a supply agreement under which NorthStar will provide Alpha-9 with actinium-225.

AuntMinnie
JUNE 10, 2024
Evans, a professor in the Department of Radiology and Biomedical Imaging at the University of California, San Francisco, was recognized for his work in biomarker discovery and radiopharmaceutical development. He will be honored at the SNMMI's annual meeting in Toronto.

AuntMinnie
NOVEMBER 4, 2024
Radiological societies have mixed views on the U.S. reduction in average Medicare payment rates compared with 2024, the CMS has estimated there will be a 0% change to reimbursement for radiology, nuclear medicine, and radiation oncology. Although the 2025 MPFS calls for an overall 2.9% Although the 2025 MPFS calls for an overall 2.9%

Imaging Technology
JANUARY 27, 2025
Our collaboration with GE HealthCare brings a practical focus on addressing well-defined clinical objectives, said Sharmila Majumdar, PhD, Research Vice Chair in the UCSF Department of Radiology & Biomedical Imaging. The team aims to standardize processes for new approaches, such as visualization of alpha-emitting radiopharmaceuticals.

Imaging Technology
JULY 9, 2024
I congratulate Marco Campione on this CEO appointment and we look forward to drawing upon his strategic, operational and broad general business expertise as we increase our global leadership throughout the diagnostic radiopharmaceutical industry,” said Fulvio Renoldi Bracco, CEO of Bracco Imaging. Bracco Imaging S.p.A., Bracco Imaging S.p.A.,

AuntMinnie
JANUARY 30, 2024
Lipton is a professor of radiology at Columbia University’s Vagelos College of Physicians and Surgeons and an affiliate professor of biomedical engineering at Columbia University. "In As such, radiopharmaceuticals consisting of a TSPO ligand coupled to a PET radioisotope serve as effective imaging agents for neuroinflammation.
Imaging Technology
FEBRUARY 21, 2024
milla1cf Wed, 02/21/2024 - 18:58 February 21, 2024 — Blue Earth Therapeutics , a Bracco company and emerging leader in the development of innovative next generation therapeutic radiopharmaceuticals, is pleased to share the publication of promising early clinical data with 177Lu-rhPSMA-10.1 177Lu-rhPSMA-10.1

AuntMinnie
APRIL 4, 2024
A decade ago, only a few radiopharmaceutical agents were used to help treat cancer patients. Hope, vice chair of clinical operations and strategy in the UCSF department of radiology, established the radioligand therapy group at the university. However, few freestanding theranostics centers exist today.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content